According to BBC, the Bulgarians claim curd was accidentally discovered around 4,000 years ago when nomadic tribes roamed the land. The nomads carried their milk in animal skins, creating a ripe environment for bacteria to grow and cause fermentation, producing yoghurt. In all likelihood, yoghurt was discovered in this way in different places at different times, and probably originated in the Middle East and Central Asia.
The concept of probiotics has led to the widespread consumption of food preparations containing probiotic microbes such as curd and yoghurt. Curd prepared at home is consumed every day in most homes in southern India.
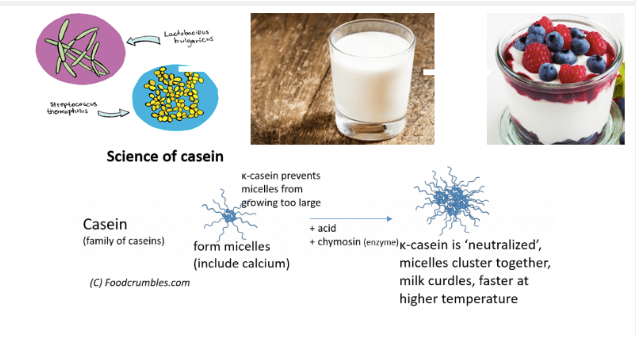
In a glass of milk, complexes of casein proteins in the form of micelles floats around. These complexes have a negative charge, so they repel each other. When we add curd inoculum to it, we are introducing loads of lactobacillus strain of bacteria which will make milk acidic.
To promote micelles clumping, you need two things: acid and heat. You can use vinegar which is commonly found at home. In chemistry, acid is a substance that produces positively charged ions which are hydrogen ion. The source of hydrogen ions is acids.
Acids are a class of positively charged molecules. When you add these to the milk, they neutralize the negatively charged casein molecules (which is what happens in a neutralization reaction in chemistry ). Without their negative charges, the casein molecules do not stay easily dissolved in the water.
This causes the casein complexes to change shape and also alter their charge. As a result, instead of repelling each other, the casein complexes coagulate or come together. As they coagulate, they capture fat and lactose along the way and get combined into a larger and larger solid, which we see as curd.
When milk is heated to a temperature of 30-40 degrees Celcius and a small amount of old curd added to it, the lactobacillus in that curd sample gets activated and multiplies. These convert the lactose into lactic acid, which imparts the sour taste to curd.
Curd making uses some sciences in it, such as the perfect temperature for the different type of curd. Curd made from milk warmed at around 70oC is thinner and tastes fresh, while curd made from milk held at 90-100degree C for 10 minutes gives thicker and creamy curd.
The more protein in milk, the thicker the curd.
This is a simple recipe to make curd at home.
- Heat 1/4 cup milk in a bowl.
- Add vinegar, salt and mix well.
- Place the starter in a bowl in which you are going to set curd and swirl the bowl.
- Pour the milk into the bowl.
- Homemade fresh and thick yoghurt is ready to be used as you wish.
Besides vinegar, apple cider, tamarind, lemon juice, or even orange juice is used as a source of acid. Now you know why acid plays an important role in our daily lives.